Cardiac Modeling

Simulation of Arrhythmia in The Ventricles
Activation wavefronts of a reentrant tachycardia in the ventricles.
Animation performed by Dr. E. Vigmond.
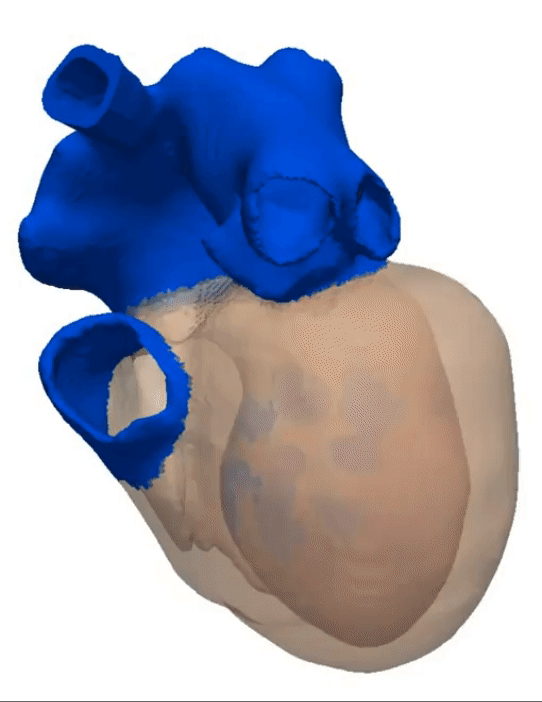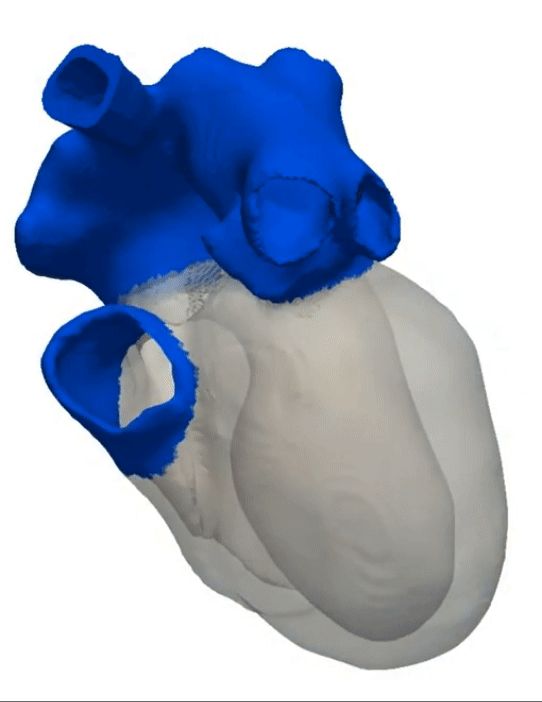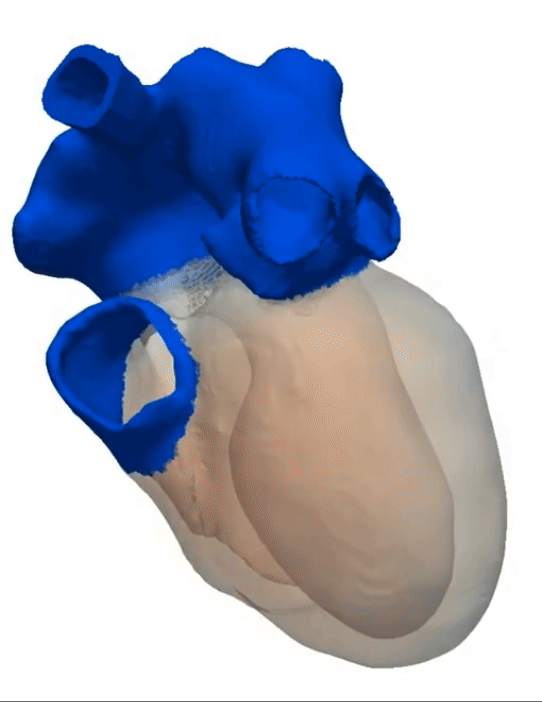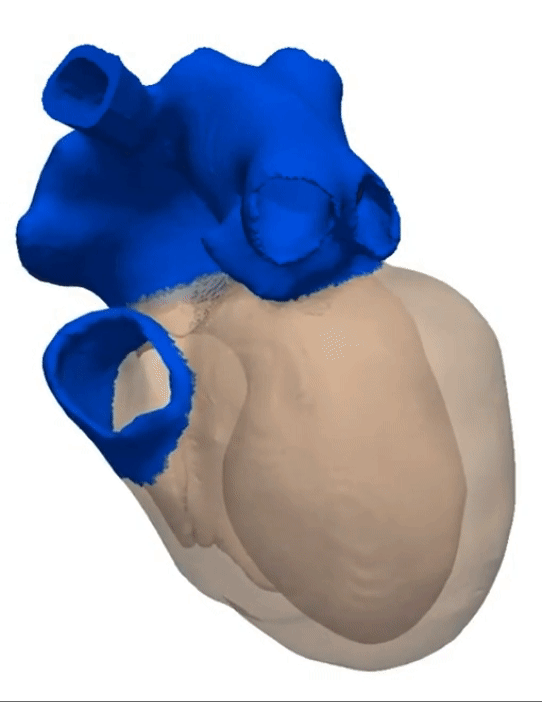
Simulation of a Normal Heart Beat
Sinus Rhythm
Animation Credits: Thanks to Dr. Gernot Plank of CCL for providing this animation.
What We Do
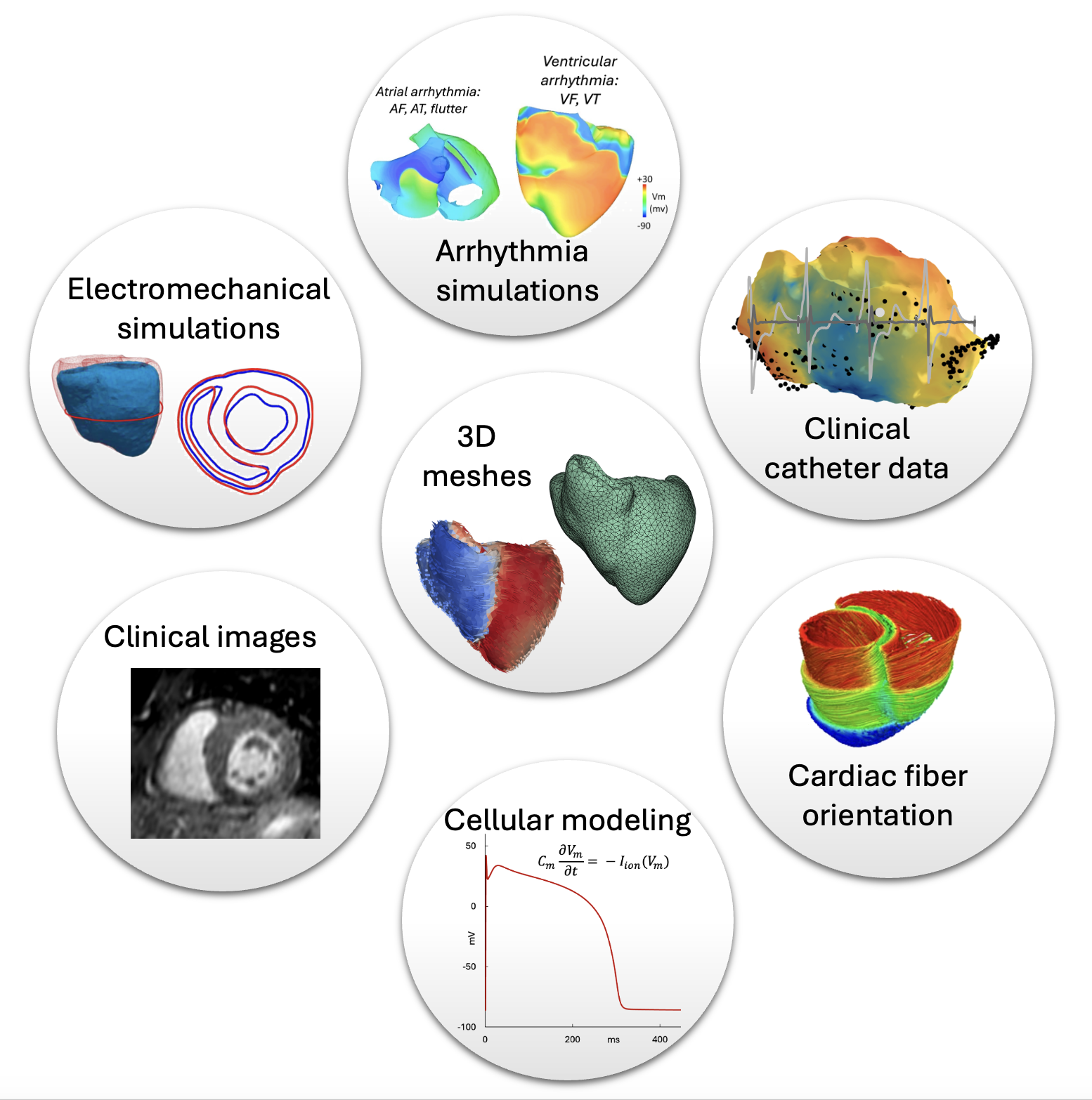
Our team is involved in several types of cardiac modeling including atrial and ventricular arrhythmia as well as electromechanical simulations reflecting the strain and stretch of the cardiac tissues as well as the fibre orientation. The simulations take into consideration mathematical models of transmembrane potentials of myocytes, the contracting cells of the heart innate with the ability to trasfer electrical signals due to ion exchange. We incorporate different clinical data ranging from CT, MRI, gadolinium enhanced MRI and microCT to produce anatomically accurate meshes. In addition, catheter data providing endocardial electrical signal data are also used to enhance the meshes.
Latest From Our Team

We were happy to host Minha Anees for her Erasmus stay in Bordeaux.
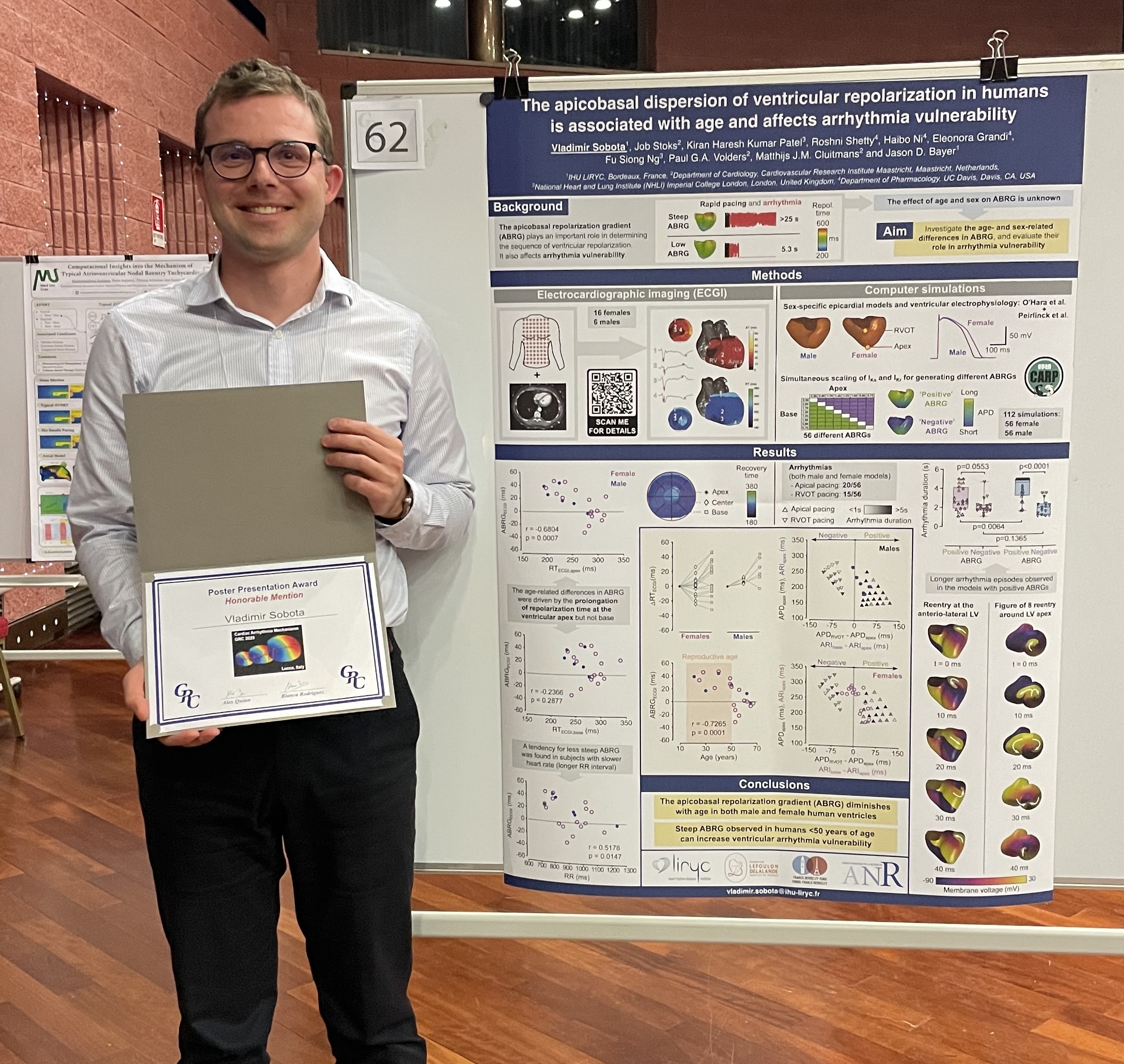
Dr. Vladimir Sobota received the Honorable Mention Poster Presentation Award at the Gordon Research Conference (GRC) on Cardiac Arrhythmia Mechanisms held in Renaissance Tuscany Il Ciocco, Lucca, Italy, from February 23 to 28, 2025. He has also been elected a chair for the 2027 Gordon Research Seminar (GRS) on Cardiac Arrhythmia Mechanisms, together with Roshni Shetty from the University of California, Davis.

Dr. Karl Magtibay giving his talk at the Gordon Research Conference (GRC) on Cardiac Arrhythmia Mechanisms held in Renaissance Tuscany Il Ciocco, Lucca, Italy, from February 23 to 28, 2025. His talk was one of the abstract-selected talks.
Featuring Some of Our Work Themes
A His bundle pacing protocol for suppressing ventricular arrhythmia maintenance and improving defibrillation efficacy
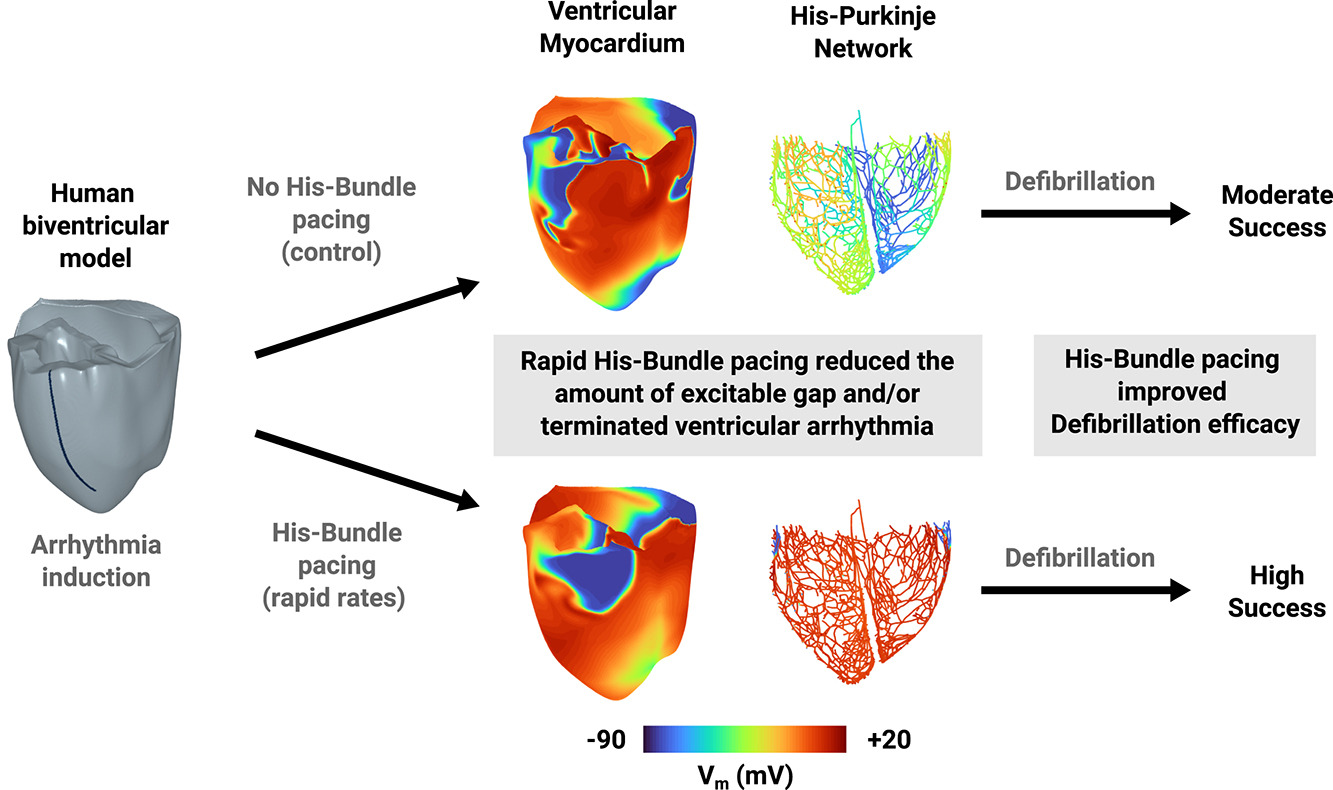
The excitable tissue between two subsequent depolarizations also known as the excitable gap (EG) in the His Purkinje Network (HPN) is critical for maintaining reentry underlying deadly ventricular arrhythmias. The aim of this work is to determine whether the rapid His bundle pacing (HBP) during reentry will reduce the amount of EG in the HPN and ventricular myocardium to suppress reentry maintenance and/or improve defibrillation efficacy. Therefore, reentry was initiated in a human biventricular virtual model with rapid line pacing followed by HBP delivered at different pacing cycle lengths (PCLs) up to 300ms. We calculated EG independently for the HPN and myocardium over each PCL and we assessed defibrillation efficacy for each PCL by stimulating myocardial surface EG with delays ranging from 0.25 to 9 s (increments of 0.25 s, n=36) after the start of HBP. We found that defibrillation was successful if reentry terminated within 1 s after EG stimulation. This defibrillation protocol was repeated without HBP. To test the approach under different pathological conditions, we repeated all the protocols in the biventricular model with right (RBBB) or left (LBBB) bundle branch block.
Conclusion: Conclusion: HBP can reduce the amount of excitable gap and suppress reentry maintenance in the HPN and myocardium. HBP can also improve the efficacy of low-energy defibrillation approaches targeting excitable myocardium. HBP during reentrant arrhythmias is a promising anti-arrhythmic and defibrillation strategy.
For more details visit https://doi.org/10.1016/j.cmpb.2024.108239
1/3
The interplay of sex-specific electrophysiology and repolarization heterogeneity governs ventricular arrhythmia sustainability in women
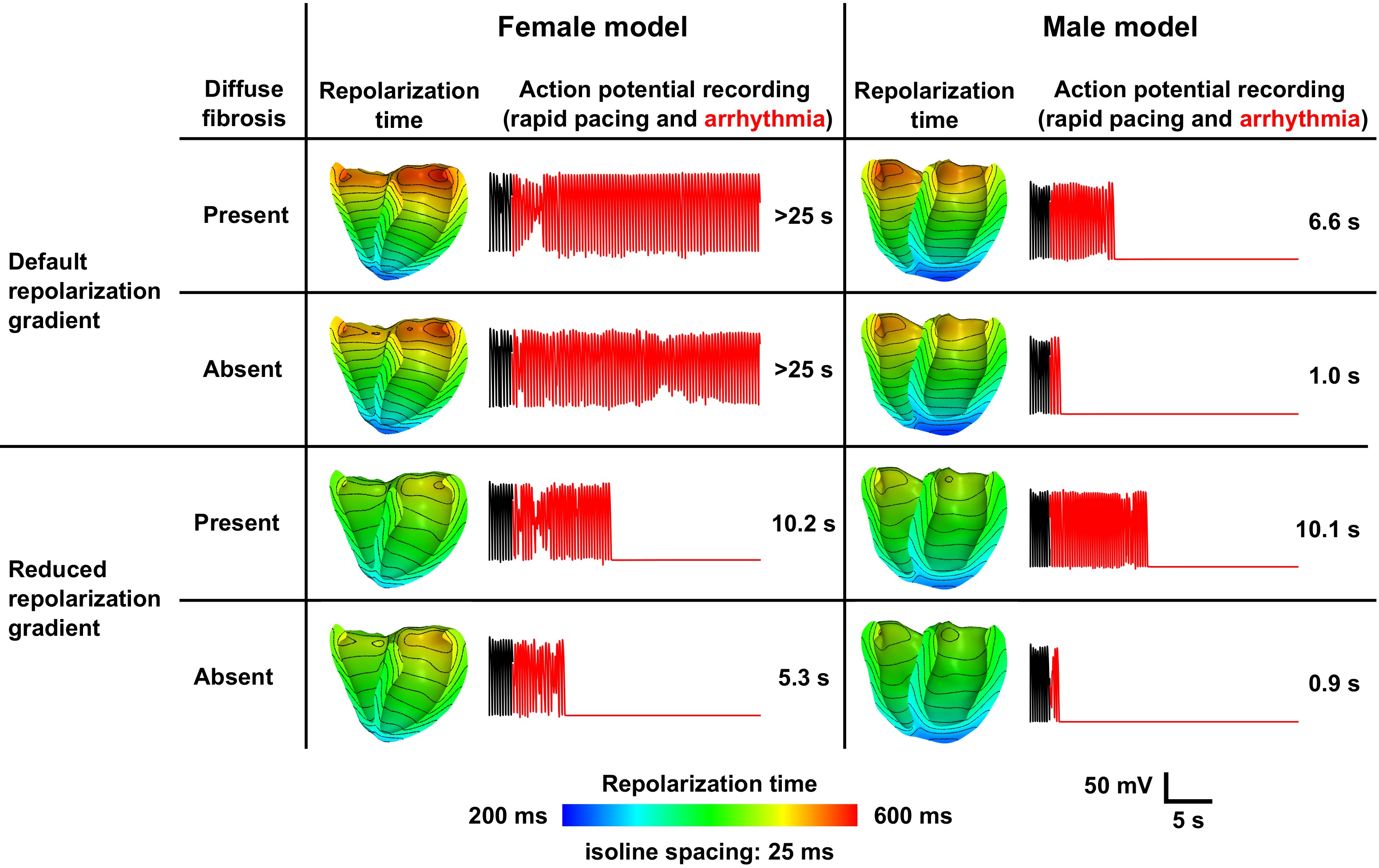
Our simulations have shown that there is a difference in electrophysiological response between genders. This study used magnetic resonance imaging (MRI) data to develop computational biventricular models of two aortic stenosis patients (a 54 years old male and a 64 years old female) before and 3 months after aortic valve replacement (AVR).
Conclusion: Longer APD and steeper apicobasal repolarization gradients in hypertrophic female ventricles resulted in sustained arrhythmia, despite their lower mass when compared to males. The absence of sustained reen- try in the post-AVR models indicates a potential electrophysiological ben- efit of AVR and encourages future clinical studies to quantify sex-specific differences in spatial repolarization heterogeneity.
For more details visit https://doi.org/10.1093/eurheartj/ehac544.2518
2/3
Meshes derived from MRI data of a male and a female subjects before and after aortic valve replacement

After AVR, ventricles have reduced ventricular mass.
For more details visit https://doi.org/10.1093/eurheartj/ehac544.2518
3/3





